
The blood microcirculatory network is where nutrients,
respiratory gases and metabolic waste products are exchanged
with the neighboring cells. Here, these components can take
unexpected routes to get from one point to another.
Whereas a simple fluid would follow the most direct route,
we show in our in-vitro experiments that red blood cells,
which are responsible for oxygenating the body, can
intermittently take side routes and remain in the network
longer than expected. These observations, backed up by an
associated modeling, raise new questions regarding hypoxia
mechanisms in organs, even under apparently healthy
conditions.
Phys. Rev. Fluids, 9,
104401 (2024). See also this
movie.
See also a
presentation of the paper at the APS Journal Club.
Seven French laboratories shared their know-how to answer a
methodological question: how should blood samples be stored
and prepared so that the mechanical response of red blood
cells is as close as possible to the physiological response?
This collaborative work was realized with the support of
GDR Mécabio, now GDR Mécabio-Santé
Biophys. J., 122, 360-373
(2023).
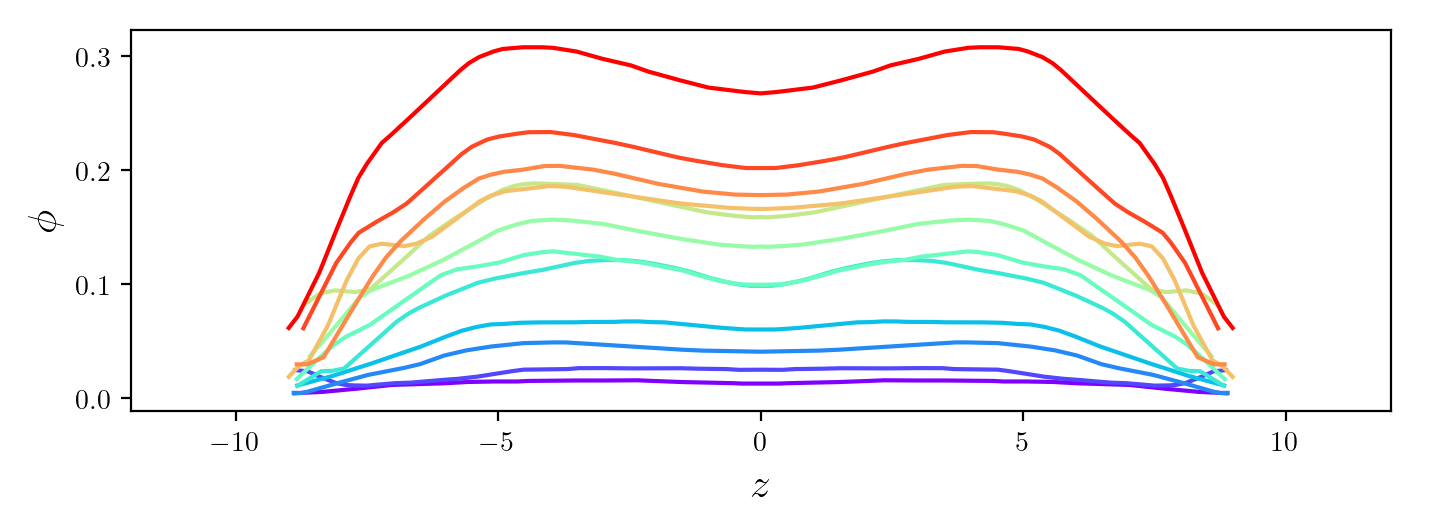
We used red blood cells as model deformable particles to
explore the rheology of a confined suspension of such
particles. In conditions where strong structuring effects
take place due to confinement, the evolution of the
effective viscosity with particle concentration shows a
remarkable succession of ranges of rapid growth and plateaus
that are associated to qualitative transitions in the
structure of the suspension.
Phys.
Fluids 34, 042013 (2022).
The structure-rheology relationship seems to crucially
depend on the mechanics of the particles and also on the 2D
or 3D nature of the problem: see Soft
Matter 20, 6677 (2024).
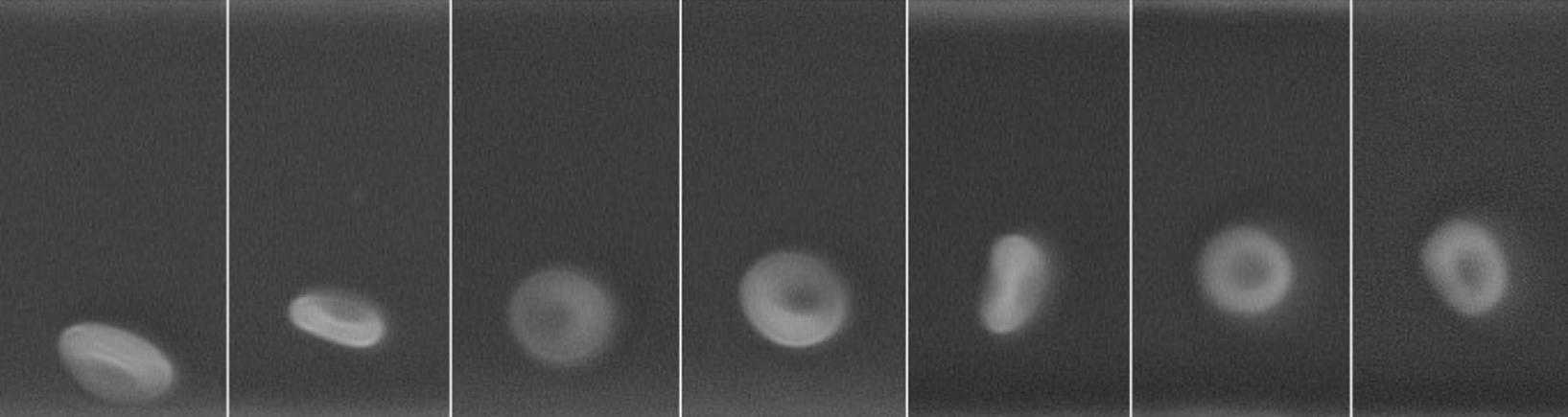
200 years ago, Poiseuille observed for the first time the
presence of depleted layers in the vicinity of the walls of
blood vessels. Through in-vitro experiments, we have
characterised the migration mechanism that is at the origin
of this phenomenon.
Microvasc. Res. 124, 30 (2019)
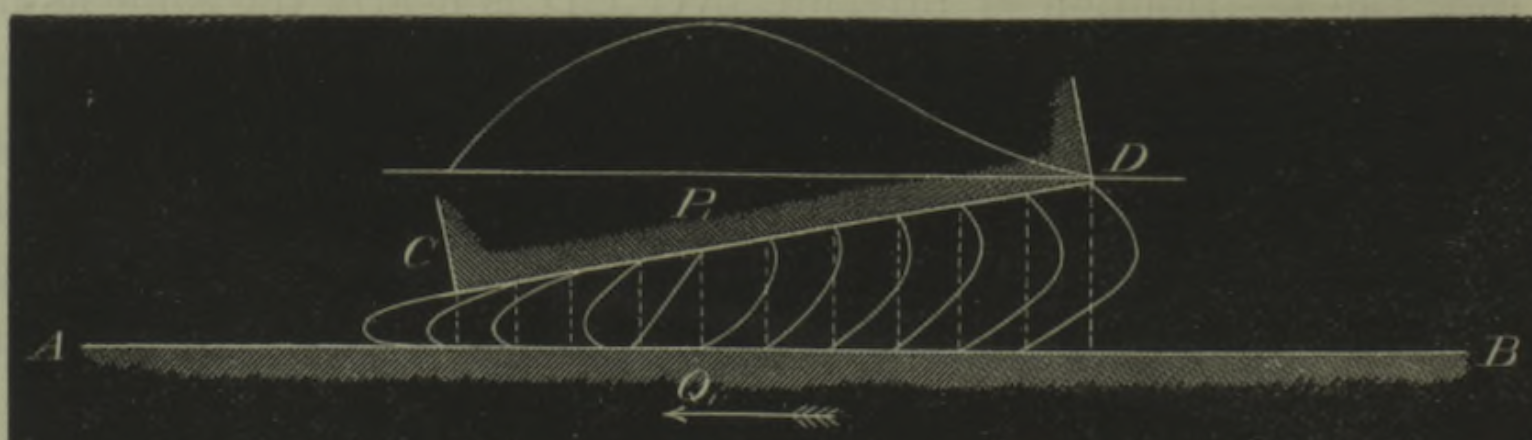
Considering a bolus of red blood cells, the dispersion in
initial lateral positions, which may be reinitialised at
bifurcations, induces strong dispersion in the residence
times within an organ. Phys. Rev.
Fluids 8, 043102 (2023)
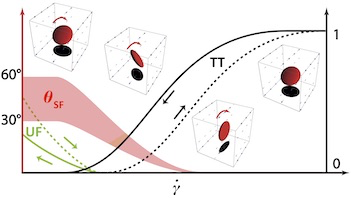
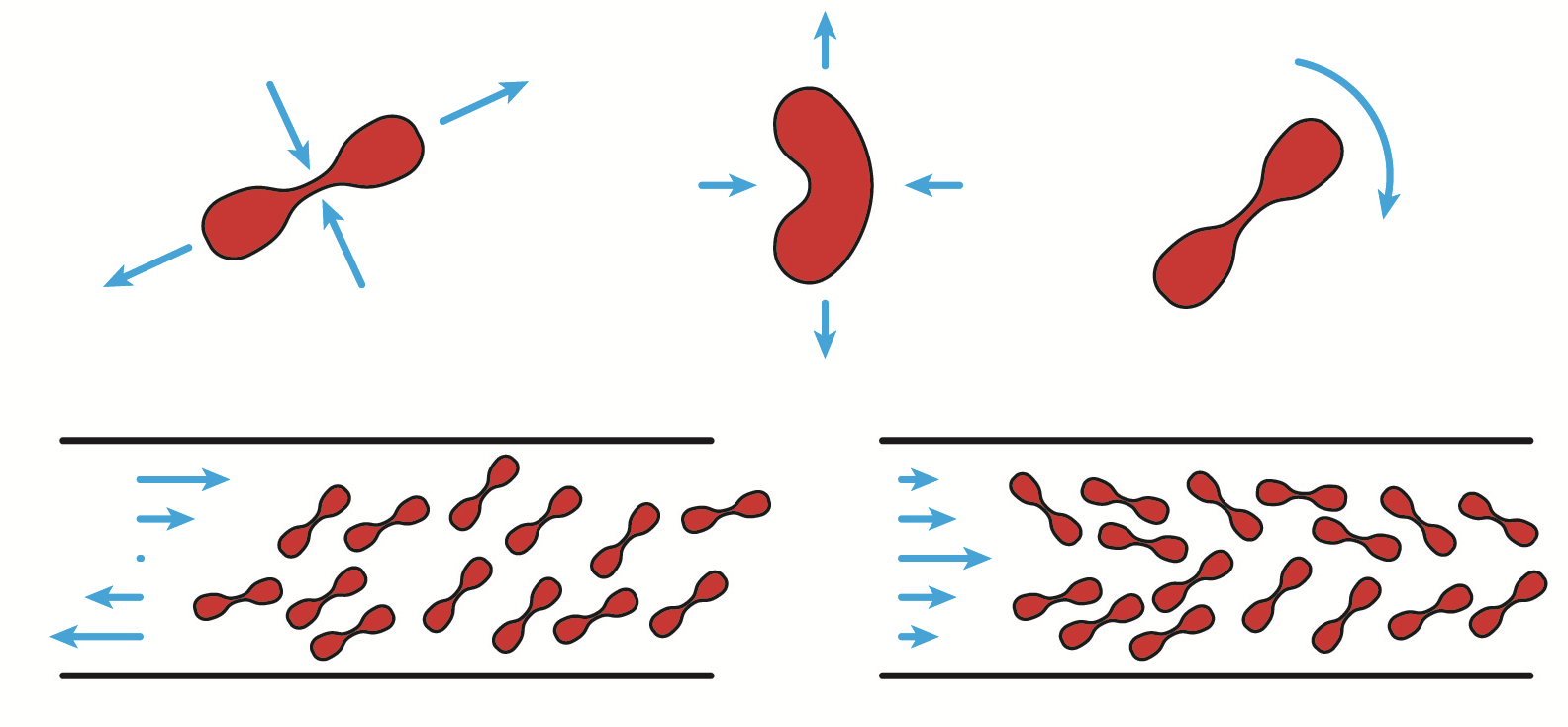
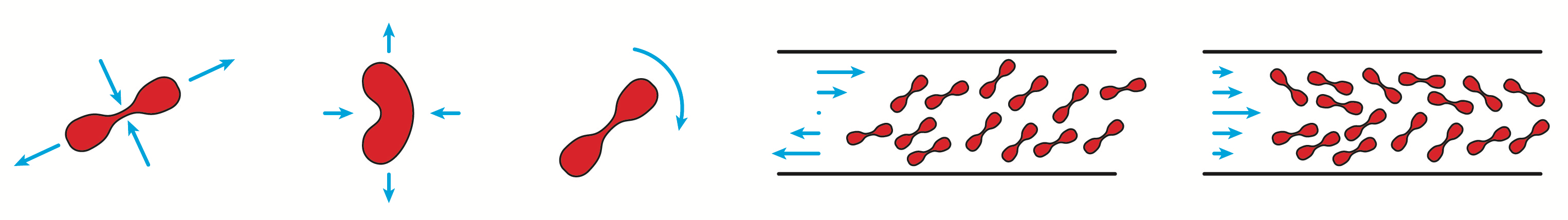
Red blood cells have a subtle mechanics which is not fully
described yet by current models. We have carried out an
extensive experimental study of their dynamics under shear
flow, which is very sensitive to their intrinsic properties
- that vary from one cell to another. This constitutes a
reference work for the validation of new models.
J. Fluid Mech. 864, 408
(2019)

At the level of a bifurcation where the flows split
unequally, red blood cells flow in such a way that the cell
concentration often increases in the high flow rate branch.
This splitting strongly depends on the upstream organization
of the cell suspension. In some cases (high confinement and
low concentration), a reverse effect is observed.
Microvasc.
Research 105, 40 (2016)

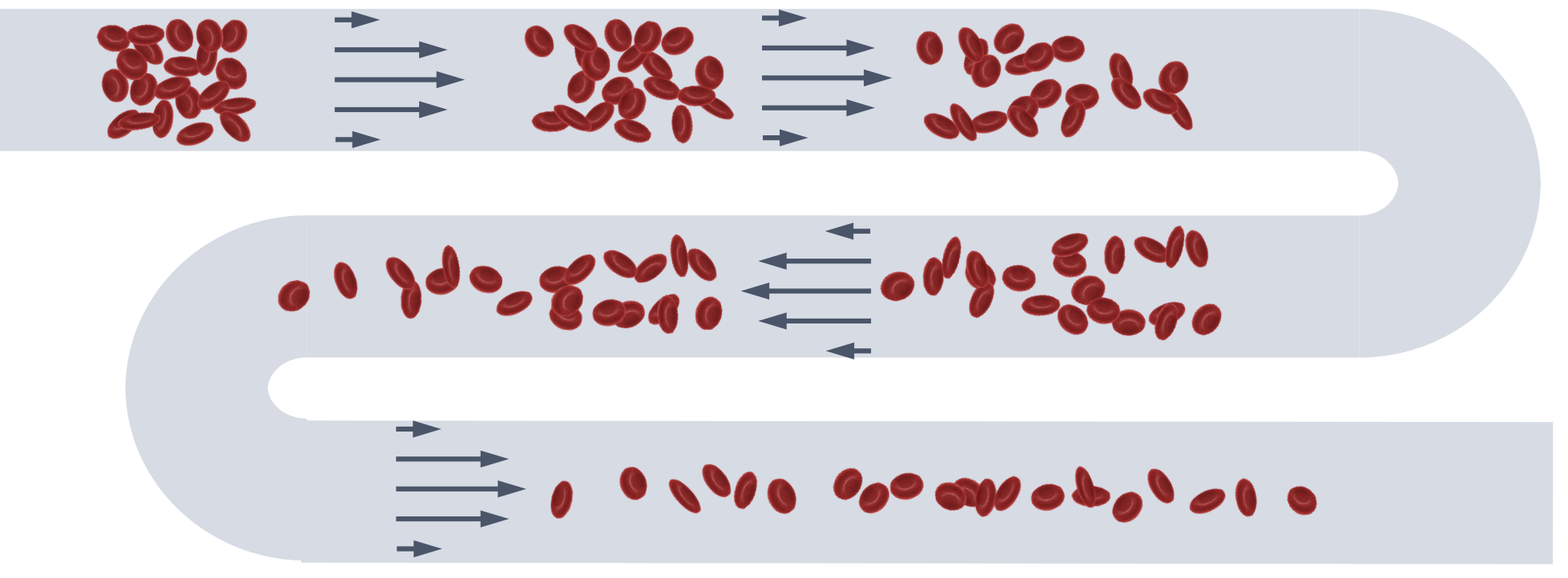
Plasma proteins cause red blood cells to form clusters
called rouleaux which are usually assumed to be
disaggregated in the circulation due to shear forces.
However, despite the large shear rates present in
microcapillaries, the presence of either fibrinogen or the
synthetic polymer dextran leads to an enhanced formation of
robust clusters.
Sci.
Rep. 4, 4348 (2014)
These clusters are initiated by hydrodynamic interactions,
which also contribute to their stabilization, in parallel to
the adhesion-induced stabilization.
Soft
Matter 12, 8235 (2016)







 orcid.org/0000-0001-5010-4148
orcid.org/0000-0001-5010-4148